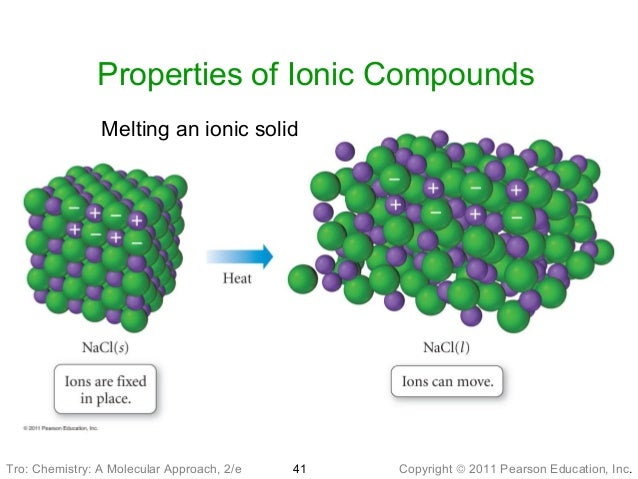
Covalent molecular compounds list
Data: 3.09.2017 / Rating: 4.7 / Views: 974Gallery of Video:
Gallery of Images:
Covalent molecular compounds list
How to write the names and formulas for ionic compounds. Learn with flashcards, games, and more for free. Compounds are organized into the following lists: List of inorganic compounds, compounds without a CH bond; List of biomolecules; See also. Chemical compounds These are examples of covalent bonds and covalent compounds. Covalent compounds also are known as molecular compounds. Organic compounds, such as carbohydrates, lipids, proteins, and nucleic acids, are all examples of molecular compounds. Covalent or molecular compounds form when elements share electrons in a covalent bond to form molecules. Molecular compounds are electrically neutral. Ionic compounds are (usually) formed when a metal reacts with a nonmetal (or a polyatomic ion). Video embeddedHere is a short list of the main properties of covalent compounds: Most covalent compounds have relatively low melting points and boiling points The chemical formula for an ionic compound represents the positive charge of the cation equals the negative charge of the anion. How can the answer be improved. Sep 14, 2016Video embeddedHow to Name Covalent Compounds. Rules for Naming Binary Covalent Compounds: A binary covalent compound is composed of two different nonmetal elements. For example, a molecule of chlorine trifluoride. If a binary compound is composed of two nonmetals, it is a covalentmolecular compound. Use the appropriate prefixes to name these simple compound List of Covalent Compounds. List Two Properties of Covalent Compounds; Related. Covalent compounds include methane, carbon dioxide and water. A covalent bond is formed when electrons are shared between compounds, and electron pairs are created as. About; FAQ's; Careers; Terms; Contact; News; Site Map; Blog; Answers; Shop; Boards; CBSE; ICSE; Hindi; Maths; Exams; Also connect with. The chemical formulas for covalent compounds are referred to as molecular formulas A chemical formula for a covalent compound. because these compounds exist as. A covalent bond, also called a molecular bond, is a chemical bond that involves the sharing of electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs, and the stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding. The chemical formula of compound can be represented with the help of atomic Covalent compounds can exist in all the three Covalent Bond Examples List Lesson 8: Naming Covalent Compounds. The purpose of this section is to specifically address the issues of chemical nomenclature. Several methods or systems for naming. molecular compounds) consist of individual covalentlybonded molecules. The covalent bonds within the molecules are very strong. Molecular compounds or covalent compounds are those in which the elements share electrons via covalent bonds. The only type of molecular compound a chemistry student is expected to be able to name is a binary covalent compound. Molecular Compounds List With this information, I hope you are thorough with the basics of molecular or covalent compounds, in terms of their identification. Use this to practice the names and formulas of covalent compounds and acids. Naming Covalent Compounds Naming B inary Ionic Compounds Polyatomic Ions Naming with Polyatomic Ions Naming with Roman Numerals Chemical Demonstration. Introduction: Covalent compounds share two electrons in forming a bond between atoms. Covalent compounds are formed only by the interactions of nonmetal atoms.
Related Images:
- The Italian
- Rambo 1 2 3 et 4 TRUEFRENCH 1080p HDRIP AC3
- The alchemist english edition
- Continental dishes recipe
- Tomtom start 20 map activation
- Samsung gamer 7 drivers
- Samsung USB Driver for Mobile Phones
- Como fazer uma mandala de cd passo a passo patchwork
- Audiosonic 12mp Camera Manual
- John Deere Tractor Light Kits
- The Dark Prophecy Pdforigin
- Delux M618 Mouse Driverzip
- Chess Strategics Vol 4 Illustrated Classic Reprint
- Bmw Z3 Manual For Sale
- Antologia poca manuel bandeira pdf
- Horror movie theme songs remix
- MSI K8n Neo4 Audio driverszip
- Tamil Dvd
- Manual Youth Lyrics
- Where Is Evaperator Drain Line On
- Ics 300 Test Fema
- Sample Cat Test Papers Year 7
- Renameman
- Case 30 4 Trencher Parts Manual
- Mele f10 pro manual pdf
- Dv4 2126tx driverszip
- Vademecum Agricola Pdf Gratis
- Ap watches royal oak offshore limited
- Kmt in the house of life pambazuka
- Short Guide To Writing About History Marius
- Franchising
- Menopausa naturalmentepdf
- Driver Audio Gigabyte GA 8i915pmzip
- Noyes whitney equation pdf
- Amsco Chapter 6 Answers
- Homemade Cuckold Orgy
- La Communication Non Violente Au Quotidien
- Land Rover Freelander Owners
- Arthamios tome 1 Chronique dun esprit vagabondpdf
- Thirukural stories in tamil pdf
- GR Wedding Album Template
- Five days until you read online free
- ScientificMethodTest7ThGrade
- The Art of Floral Designpdf
- Modern Psikoloji Tarihi Schultz Pdf
- Pengertian siklus hidrologi tertutup dan terbuka
- Steels Microstructure and Properties
- 2013 Sherco St 250 Wiring Diagram
- Mecanica De Suelos Juarez Badillo Pdf Tomo 1
- 1953 Ford Jubilee Tractor Manuals
- Manual Motor Weg W22
- Giuseppe Veronese e i fondamenti della geometriapdf
- Manual De Ajuste De Motor Vw 1600
- Magnetismo Pessoal Turnbull Pdf
- La missione di Michelepdf
- Gem ws1 oriental manual
- Sadlier Oxford Math Grade 7 Answers
- Hebrew from scratch part 1 textbook
- Samsung Le26a457c1d Tv Service Manual Download
- Advanced Cardiovascular Life Support Test Answers
- Pidion Bip 6000 Driver for Windows 7zip
- Caesar De Bello Gallico Book 7 Summary
- Kleine kwalen in de huisartsenpraktijk
- Flash game aqua energizer fg
- La Parure Des Abdal Pdf
- Serie rick hunter
- Principles Of Electronics Analog And Digital
- DansPornAndTaboo Dee Dee Lynn Little Brat
- Lekturehilfen Patrick Suskind Das Parfum
- Sadman search
- Manuale operativo di criminologiapdf
- Manual De ServiTv Toshiba 2177
- Livro A Arte De Ler Mentes Pdf Download Gratis
- Assassins Creed Revelations Skidrow
- Partes de un proyecto de investigacion wikipedia
- Biology Concepts And Connections 4Th Edition Test Bank
- Descargar La Dieta Del Genotipo Pdf Gratis
- Net Computer Nc120 Driver Windows 7zip
- Mixed exercises tenses grammar with answers key
- Alcatel USB Driver for Windows XPzip
- All adobe portable collections 11in1
- Pull Push 2
- Enemy of My Enemymp3
- Geometry For Enjoyment Challenge New Edition